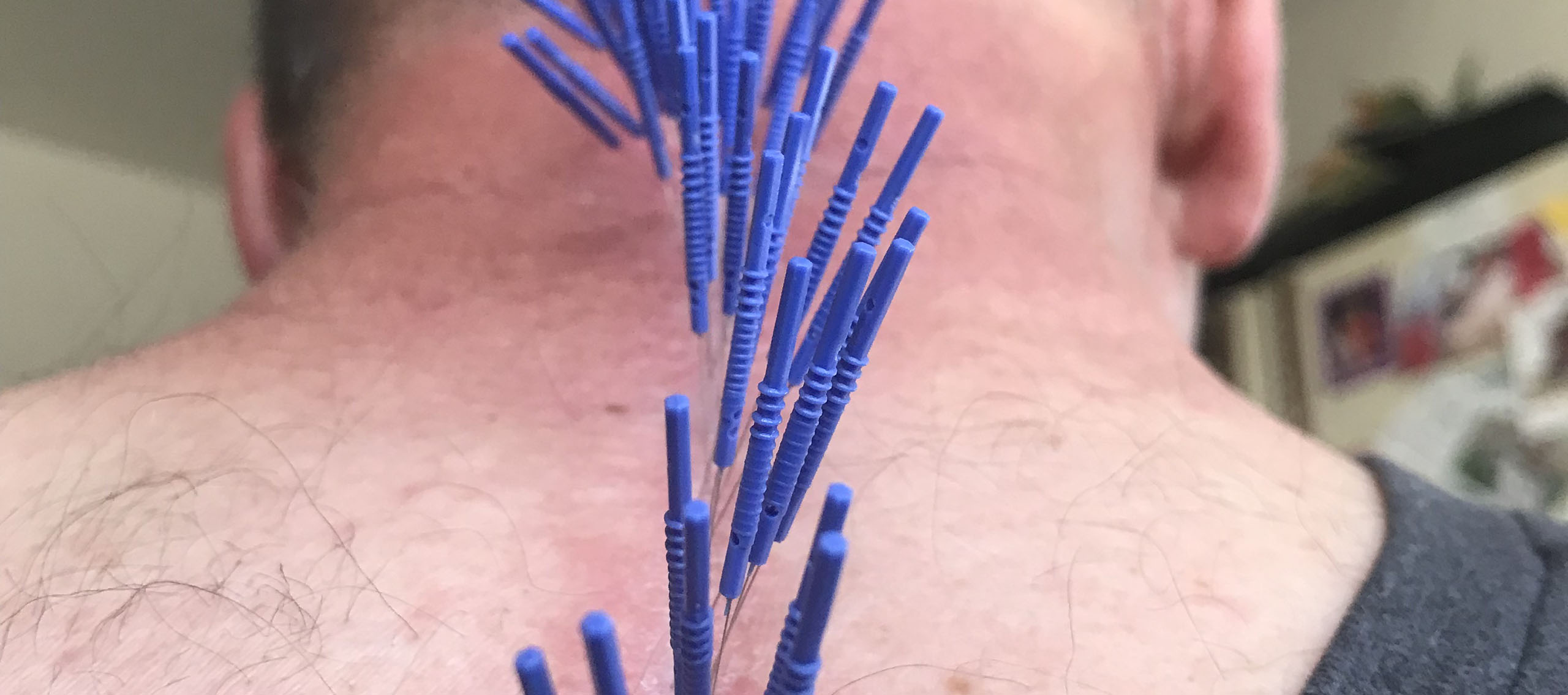
How Does Dry Needling Work
Everything Dry Needling
Everything Dry Needling
How Does Dry Needling Work? How is it Different from Acupuncture? How to Think About & Treat the Autonomic Nervous System with Dry Needling to Improve Autonomic Nervous System Homeostasis: The Key to Health
Table of Contents
- What is Dry Needling?
- How Does Dry Needling Normalize Tissue Length?
- Dry Needling Safety
- Electrical Auricular Vagus Nerve Dry Needling
- Auricular Vagus Nerve Needling IS NOT Acupuncture
- How to Limit Initial Sympathetic Autonomic Stimulation Following Needle Placement to Avoid Vasovagal Response and Patient Discomfort During Dry Needling Treatment
- Should I Utilize the “Pistoning” Technique While Performing Dry Needling?
- References
Table of Contents
- What is Dry Needling?
- How Does Dry Needling Normalize Tissue Length?
- Dry Needling Safety
- Electrical Auricular Vagus Nerve Dry Needling
- Auricular Vagus Nerve Needling IS NOT Acupuncture
- How to Limit Initial Sympathetic Autonomic Stimulation Following Needle Placement to Avoid Vasovagal Response and Patient Discomfort During Dry Needling Treatment
- Should I Utilize the “Pistoning” Technique While Performing Dry Needling?
- References
What is Dry Needling?
One of the most common questions I get from students is, “How do I describe how dry needling works?” I have compiled and combined numerous past articles I have written on the subject, along with some new information, into the following article. Let me know if anyone has any questions.
The overwhelming majority of Americans are living with chronic sympathetic nervous system hyperactivity. This is real bad. Like eating a spoonful of hot, rancid mayonnaise… Yes, that bad. If the autonomic nervous system is dysregulated into sympathetic hyperactivity for more than a few days, all of our internal functions begin to degrade. Blood pressure elevates, heart rate increases, PVC’s or arrhythmias may begin, nerve conduction velocity goes down, oxygen saturation decreases, liver filtration slows down, anxiety increases, pain increases, muscles shorten, tissue hypoxia gets worse, and a lot of other not-so-great stuff happens. When the sympathetic autonomic nervous system goes up, the parasympathetic autonomic nervous system gets reciprocally inhibited, leading to generalized widespread panic and dysfunction of our neuromusculoskeletal systems.
I am not going to spend much time on the easiest, best overall ways to improve your healthspan and lifespan: proper diet, consistent exercise, staying in shape, and good sleep. Doing these simple things increases lifespan by over a decade, by the way. I highly recommend Dr. Peter Attia, Dr. Mark Hyman, Dr. Andy Galpin, and Dr. Andrew Huberman’s podcasts to obtain the most up-to-date scientific knowledge on these topics. They are all awesome and are excellent at explaining these complicated topics in understandable ways.
I spend a lot of time speaking to my patients about the importance of these factors. However, the simple fact of the matter is, that most people do not eat well, do not exercise, never will, and subsequently, are in poor physical and mental condition. Therefore, it behooves us to explore alternative treatments to naturally regulate the autonomic nervous system toward homeostasis, removing inhibition and facilitating our awesome, innate ability to function and heal.
It takes about 8 weeks of strenuous exercise, 3 times a week, to begin building new sarcomeres in parallel and in series to make muscles physically larger and add muscle mass. That is 24 visits of physical therapy. Per numerous studies, the average number of physical therapy visits insurance approved per patient, in 2022, was 8-12, or so. This makes it scientifically impossible to add muscle tissue, sarcomeres, onto existing muscles during a typical physical therapy treatment plan of care, even if all you do is exercise.
The overwhelming majority of physical therapy patients do not perform their home exercises and will never exercise unless they are in the clinic with you instructing them. 79% of the US population is overweight, obese, or morbidly obese, for goodness sake. For this reason, exercise is the least useful tool physical therapists have at their disposal. Unfortunately, exercise is the most commonly performed treatment in outpatient physical therapy settings. Now, I am not saying exercise is bad, it is absolutely vital to mental and physical well-being. What I am saying is, secondary to restrictions imposed by the lovely, corrupt insurance companies, performing 8-12 sessions of exercise is doing nothing for you long term. All it’s doing is temporarily making the autonomic nervous system more homeostatic, secondary to the simple act of moving around and stressing your tissues, which most people never do on their own. Any strength gains you see are neurophysiologic improvements, which rapidly diminish as soon as the patient is done with physical therapy, if they do not continue to exercise on their own, which they do not, in general.
The most potent treatment we can provide our patients is removing as much tissue pathology throughout the body as possible, while at the same time specifically targeting the parasympathetic portion of the autonomic nervous system. This maximally induces autonomic nervous system homeostasis, which should be the overall goal of treatment. This allows the mind and body full access to the awesome, mind-boggling, innate healing powers they possess. The problem is that these magical healing powers become concealed from use, and are often hijacked and used against us (cancer is one example), secondary to chronic sympathetic autonomic hyperactivity. The most powerful tool physical therapists have at their disposal, one of the most powerful tools in all of medicine, in my opinion, to regulate the autonomic nervous system toward homeostasis is the thoughtful implementation of dry needling, especially when combined with joint manipulation.
How Does Dry Needling Normalize Tissue Length?
So, DN works on 2 primary levels. Mechanical and neurologic, although I believe the mechanical changes are primarily driven by the nervous systems changes induced by needling. There are a bunch of things that need to happen for a muscle to contract, but there are a few primary things that need to occur. Let’s think about this in terms of a sarcomere and the actin-myosin cross-bridge formation.
Normal Skeletal Muscle Contraction
The primary things that need to happen for an actin-myosin cross-bridge to form are: Acetylcholine, a neuromodulator, needs to cross the pre-synaptic cleft to the post-synaptic cleft, which signals the Sodium-Potassium pump to depolarize the cell. Troponin needs to be present inside the cell and calcium needs to be released from the sarcoplasmic reticulum, which is already intracellular. Troponin C and calcium bind together, move tropomyosin out of the way, and form the cross-bridge site for the actin-myosin attachment.
Normal Skeletal Muscle Relaxation
The primary things that need to happen to disconnect the actin-myosin cross-bridge are: Acetylcholine esterase needs to be present in sufficient concentration to gobble up all the acetylcholine to stimulate the sodium-potassium pump to repolarize the cell. Tropomyosin enters the cell and the calcium-troponin bond dissolves. Calcium gets reabsorbed into the sarcoplasmic reticulum and tropomyosin blocks the actin-myosin binding site.
Note: Remember, normal resting muscle cell potential for skeletal muscle is about -70 millivolts, or so. An action potential typically occurs at +40 millivolts, or so. Also remember, the vast majority of PT patients present with sympathetic hyperactivity of both the ANS and CNS. With hyperactive sympathetics, the resting potential of the sarcomere is less negative, maybe at -50 millivolts, and the action potential is less positive, maybe around +30 millivolts. In this instance, the electrical potential of the cell needs to change far less to stimulate an action potential. This is one of the reasons that sympathetically hyperactive sarcomeres produce spontaneous electrical activity.
Note: We know very little about biology. Unfortunately, many things we have known about for decades, very important things, are still not taught in medical school. Here is an excellent example of a super important protein involved in the actin-myosin contraction and relaxation mechanism, the protein Titin. Discovered over 3 decades ago.
The Sarcomeric Spring Protein Titin: Biophysical Properties, Molecular Mechanisms, and Genetic Mutations Associated with Heart Failure and Cardiomyopathy: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8522185/
Abnormal Skeletal Muscle Contraction & Relaxation Secondary to Sympathetic Hyperactivity
Hyperactive sympathetics lead to excessive acetylcholine production / release, diminished acetylcholine esterase production / release, along with excessive intracellular calcium concentration. These factors make it next to impossible for the actin-myosin cross-bridge to disconnect. A detrimental feedback loop ensues where the muscles hurt, they contract more, become more hypoxic, more nasty chemicals accumulate, many of which are pain amplifiers, the muscle cannot relax, it hurts more, and so on.
Another thing that happens with chronically hyperactive sympathetics is, rather than just the post synaptic cleft being receptive to acetylcholine, the entire epimysium (muscle lining) becomes receptive to acetylcholine. With excessive concentrations of acetylcholine and the entire epimysium becoming receptive to it, a slow, chemical shortening of muscles begins. This is not active, spontaneous electrical contraction, it is a chemical contraction, which may not show up on an EMG.
Hyperactive sympathetics also dysregulate the hypothalamic-pituitary-adrenal (HPA) axis and the gut-brain-axis (enteric nervous system + brain), both part of the ANS, along with the trigeminocervical complex. If these axes and complexes are dysregulated, all of our hormones, peptides, neuromodulators, immune systems, etc., become dysregulated and malfunction. This can lead to just about any mental or physical impairment.
Dry needling is, without question, the most powerful tool Physical Therapists have at their disposal to quickly and effectively regulate the central and autonomic nervous systems toward homeostasis, which, in turn, regulates our muscle health. Without autonomic homeostasis, nothing in our body works properly. Remember, the overall effect of needling, almost regardless of how it is performed, results in sympathetic depression below baseline and parasympathetic elevation above baseline. This works out well for us since basically all physical therapy patients present with sympathetic hyperactivity. This works especially well if you specifically target the parasympathetics with needles, along with whatever else you are needling.
This decreases the amplitude and duration of the initial sympathetic spike following needle insertion, which typically lasts about 15 minutes, according to numerous heart rate variability and microneurography (gold standard ANS test) studies. If parasympathetic-specific needles are implemented, the body and mind are allowed more treatment time in parasympathetic dominance, making treatment more effective, lasting, and comfortable.
Now, these are a few of the things we think we have a decent grasp on. However, to remind you, we don’t even know for sure how a muscle contracts! It is still called the sliding filament theory for a reason. It is still a theory. I always think about this; if it was possible to quantitatively assess all the medical knowledge available out there in the ether, I bet we know less than 5% of the whole. If you really think about it, we have very few 100% fixes for any medical condition. Most of the treatments we have today are just Band-Aids that mask the symptoms of the underlying problem. They don’t actually address the cause of the problem, limiting their efficacy and maximizing side effects. This, in part, is because the pharmaceutical companies would rather not have any 100% cures for diseases. It is much more profitable to keep people partially sick for life. That way, you give them medicines that only mask symptoms, and if the medicine causes side effects, you get more medicine to counter the side effects of the original medicine you were taking. It’s disgusting. Unfortunately, the one thing that rarely gets addressed, a key aspect of medicine, is treating everyone as an individual and addressing the root cause of the problem, along with the symptoms. Not just the symptoms.
Every tiny piece of normalized soft tissue, every normalized joint, every millimeter of normalized capsular tissue, removes stress from the autonomic nervous system and gives energy back to it, which improves cerebral blood perfusion and helps to restore normal neuronal activity. If you restore enough energy to the autonomic nervous system, the mind and body break out of their inhibited state and are able to direct ample resources to fix ongoing problems. I think of it as running an antivirus program on the computer. Barring specific, ongoing injury, almost all “idiopathic” impairments have a root cause of autonomic nervous system energy deficiency and a departure from homeostasis. Rather than trying to fix specific impairments, if we try to fix the autonomic nervous system, we allow the brain and body to fix themselves. Trust me, our systems know how to fix themselves far better than we ever will. We just need to remove the stuff inhibiting them from doing so.
If the autonomic nervous system goes over your individual threshold for sympathetic activity, it loses its ability to self-regulate. This leads to our systems becoming energy deficient. Our cells start producing less energy per molecule of glucose processed, our body is less efficient at breaking down food to acquire nutrients, our system and cells run out of energy, and everything gets hypoxic and inflamed. The mitochondria and other organelles in our cells run out of ATP, NADH, NAD+, O2, and other stuff, then our systems have to decide where to send and use the available energy because we no longer have enough to properly deal with everything going on. This is what leads to cancer, autoimmunity, diabetes, hearing loss, chronic pain, mental health problems, and any other nasty thing you can think about.
I regard needling as a brain treatment. A path to restore our innate superpowers. We are just using the soft tissues and nerves throughout the body as access points to affect various locations in the brain. The needle is a significant-enough amount of direct tissue stimulation to induce incredible homeostatic and neuroplastic alterations in the brain. The speed of complete recovery from unusual, random, “idiopathic” neurologic impairments I have observed consistently over my ten-year career never ceases to amaze me. Particularly with people who are not in good shape, do not eat well, do not supplement, and do not exercise. Nevertheless, thoughtfully performed needling leads the autonomic nervous system back from the abyss, into a realm where it can once again effectively self-regulate and maintain homeostasis.
Dry needling is one of the few treatments in Medical-Land that addresses both the cause of the problem and the symptoms, for just about any impairment out there. Again, I don’t know of any medical impairment that is not helped by improved CNS / ANS homeostasis, and needling is the most powerful tool physical therapists have at their disposal to regulate the autonomic nervous system toward homeostasis.

Dry Needling Safety
Dry needling is about as safe a medical treatment as there is, if you are taught properly. It is certainly safer than taking just about any medication. Don’t forget, Ibuprofen has DEATH listed numerous times in the full side-effects list. I have been needling my entire career, coming up on 10 years, and have never had a negative experience. It is very easy to teach DPT’s how to avoid the lungs and other organs. Out of the organ field, the worst thing you are going to potentially do to a patient is give them a bruise or a zinger. It is very difficult to hit neurovasculature, even if you are trying to.
The needle pushes a fluid/pressure bubble in front of it as it passes through living tissue. This alerts the patient when you are approaching a nerve, and makes it easy to never hit a nerve. Major neurovasculature typically runs together, which is nice for us because the nerve will almost always alert the patient prior to ever puncturing a nerve, artery, or vein. There has been a lot of medical research looking at what causes damage from injections. Mind you, a blood draw needle is about 10 times as big as the needles I use, and it is hollow. Basically, the only thing that typically causes lasting neurovascular damage is the injection itself, if it creates too much pressure and ruptures stuff from the inside.
It’s important to take a step back and think about this stuff. When you get blood drawn, the person taking your blood typically has a 6-month certificate in phlebotomy, and they are intentionally poking vessels directly over a major nerve, the median. I mean no offense by this. MD’s take pieces of organs to biopsy them all the time and the organs don’t fail. Our needles are so small that even if you did hit something once, you most likely would never know you did anything. It’s a 0.2 mm hole that seals immediately when the needle is removed. This is a primary reason to not “piston.” It is not the best for the nervous system and it dramatically increases the risk of anything significant occurring. More on this in a bit.
Dry Needling Risk vs Reward: Pneumothorax & Infection
Short Answer: If properly trained in needling, there is almost zero risk of pneumothorax and infection. I have been needling for almost 10 years, have put hundreds of thousands of needles into people, and have never had a negative experience.
Dry needling, if properly trained, is super safe and super effective. Those are scientific terms. In layman’s terms, needling is totally awesome. It is, without question, the most powerful tool physical therapists have at their disposal. There is no medical condition that does not benefit from improved autonomic nervous system homeostasis, that I know of, and thoughtful needling regulates the autonomic nervous system toward homeostasis. However, many medical doctors and other medical professionals who refer patients to therapy, are unfamiliar with dry needling and are understandably, although unduly, concerned with the risk of pneumothorax and infection. In relation to needling, Infection is typically the primary concern of physicians following surgery.
Needling & Infection Following Surgery
Needling reduces the risk of infection following surgery. Yes, I meant to say that. Needling reduces the risk of infection following surgery. When you take a step back and think about the needle itself, and the neurophysiologic responses induced by needling, this is apparent.
The typical needle I use is 0.2 mm in diameter. It is a solid, sterile piece of surgical steel. It does not take a piece of skin into the underlying tissue like a hollow needle does. Studies have been performed to see the amount of infectious-causing material one of these needles can bring into the body, and it is orders of magnitude less than the smallest amount needed to potentially cause an infection. The improved immune function secondary to improved autonomic homeostasis stimulated by thoughtful needling far outweighs any minuscule increased risk of infection simply utilizing a needle entails.
Remember, the overall effect of needling is a reduction of sympathetic autonomic activity and elevation of parasympathetic autonomic activity. This happens almost regardless of how you needle. If you specifically target the parasympathetics, along with whatever else you are treating, this homeostatic autonomic response is significantly strengthened. Trauma, which is what surgery is, causes sympathetic hyperactivity. Evolutionarily speaking, sympathetic hyperactivity (including our fight-or-flight response) evolved to help protect our bodies from further immediate damage. A bunch of epinephrine, cortisol, cytokines, glutamate, and other stuff is released from the hypothalamic-pituitary-adrenal (HPA) axis and other locations, the enteric nervous system malfunctions, peripheral vasoconstriction occurs, and our muscles get tight to prevent further tissue damage. This inhibits ideal healing, especially considering we are typically in a safe and controlled environment following surgery and are not in immediate threat of further injury.
If this immediate sympathetic autonomic hyperactive response is not controlled, detrimental autonomic feedback loops begin to form, leading to all sorts of issues, increased risk of infection being one of them. Remember, research indicates that over 90% of post-surgical infections occur because of some infectious agent entering the body during surgery. The best way to make sure that infectious-causing agent actually turns into an infection, is to dramatically reduce blood flow. This allows for replication of the infectious agent without intervention from enough new white blood cells and other infection-fighting agents. Our body is really good at fighting off infection with proper fluid circulation and autonomic homeostasis. Both of these factors are severely limited following surgery, unless proper intervention is provided.
The gut-brain axis (enteric nervous system & brain, connected by the vagus nerve) and the HPA axis play huge roles in regulating immune function. Following surgery, both of these axes become dysfunctional. Employing thoughtful needling, specifically targeting the parasympathetic autonomic nervous system, reduces sympathetic autonomic hyperactivity and nudges the autonomic nervous system toward homeostasis. Autonomic homeostasis allows maximal function of the HPA and gut-brain axis, improving immune system response and reducing the risk of infection following surgery. Vagus nerve needling, the auricular branch, along with needling in general, has been clinically proven to significantly improve body wide vasodilation and elicit marked sympathetic depression, parasympathetic elevation, and autonomic homeostasis compared to baseline. This dramatically improves immune function and the body’s ability to detect and fight off foreign, infectious agents.
The local neurophysiologic effects of implementing needling treatments relatively close to surgical repairs, immediately following surgery, reduces the risk of infection. Following surgery, there is good blood flowing to the surgical site, but not flowing through the surgical site. Super tight muscles can restrict venous blood flow. Veins don’t have thick walls like arteries do, so they can get squished. This squishing, from muscle contraction, is a primary way blood gets returned to the heart, along with the one-way valves inside veins. This is sometimes referred to as “milking veins.”
If muscles produce trigger points and become hypertonic, as they do following surgery, the squishiness of veins is their downfall. Weaklings! Muscles compress them and blood flow slows down. The valves in the veins that typically stop blood going away from the heart, get stuck open and allow blood to backflow. This is the primary issue with erectile dysfunction, as well. Then, rather than muscles contracting and relaxing, you just have grumpy, contracted, hypoxic, rigid muscle. This leads to decreased venous blood flow. This is one of the reasons edema and swelling occur, along with peripheral vasoconstriction secondary to sympathetic hyperactivity.
Hypoxia and poor blood flow through a surgical site increase the risk of infection. Stagnant, old blood that gets stuck in tissues near surgical sites does not have healthy white blood cells or other nutrients to fight off infection. Needling stimulates significant increases in vascular and microvascular circulation, secondary to sympathetic depression, parasympathetic elevation, calcitonin-gene-related-peptide release (a potent microvascular dilator), sarcomere relaxation, and other known and unknown processes.
Trigger points are hypercontracted groups of sarcomeres that can occlude just about all blood flowing through them. This leads to a buildup of pain-amplifying and potentially infectious agents. Like infection ninjas… Trigger points also shorten muscles. Short muscles hurt, causing more sympathetic hyperactivity and vasoconstriction, hindering HPA and gut-brain axis function, harming our immune system, and increasing the likelihood of infection. Needling is the best way that exists on the planet to rapidly and effectively remove trigger points and normalize muscle length, thus regulating the autonomic nervous toward homeostasis, reducing the risk of infection following surgery. This effect is maximized by concomitantly connecting the parasympathetic-dominant portions of the body to each other with 1 Hz microcurrent.
Dry Needling & Pneumothorax
If properly trained, the risk of needle-induced pneumothorax is almost, basically zero. Again, I have placed hundreds of thousands of needles into people without ever having any type of negative experience, infection and pneumothorax included. The primary reason for needle-induced pneumothorax is error in judgment. Trying to do something you should not, without the assistance of imaging guidance. This makes not causing a pneumothorax super easy. Don’t try to do things you shouldn’t. I know that sounds kinda dumb, but it really is true. The trick is, however, understanding what you shouldn’t do in the first place.
There are a few rules of thumb to go by when needling over the pleural field, and if these are followed, you will never have a problem. Osteopaths have done some really cool studies looking at things like the average vertebral size in the pleural field and how far it is on average from the surface of the skin to the surface of the pleura. Along with a few technical instructions, simply understanding the typical and atypical anatomy of the plural field and utilizing solid judgment on an individual patient basis, allows you to needle in the pleural field with as close to a zero percent chance of pneumothorax as possible.
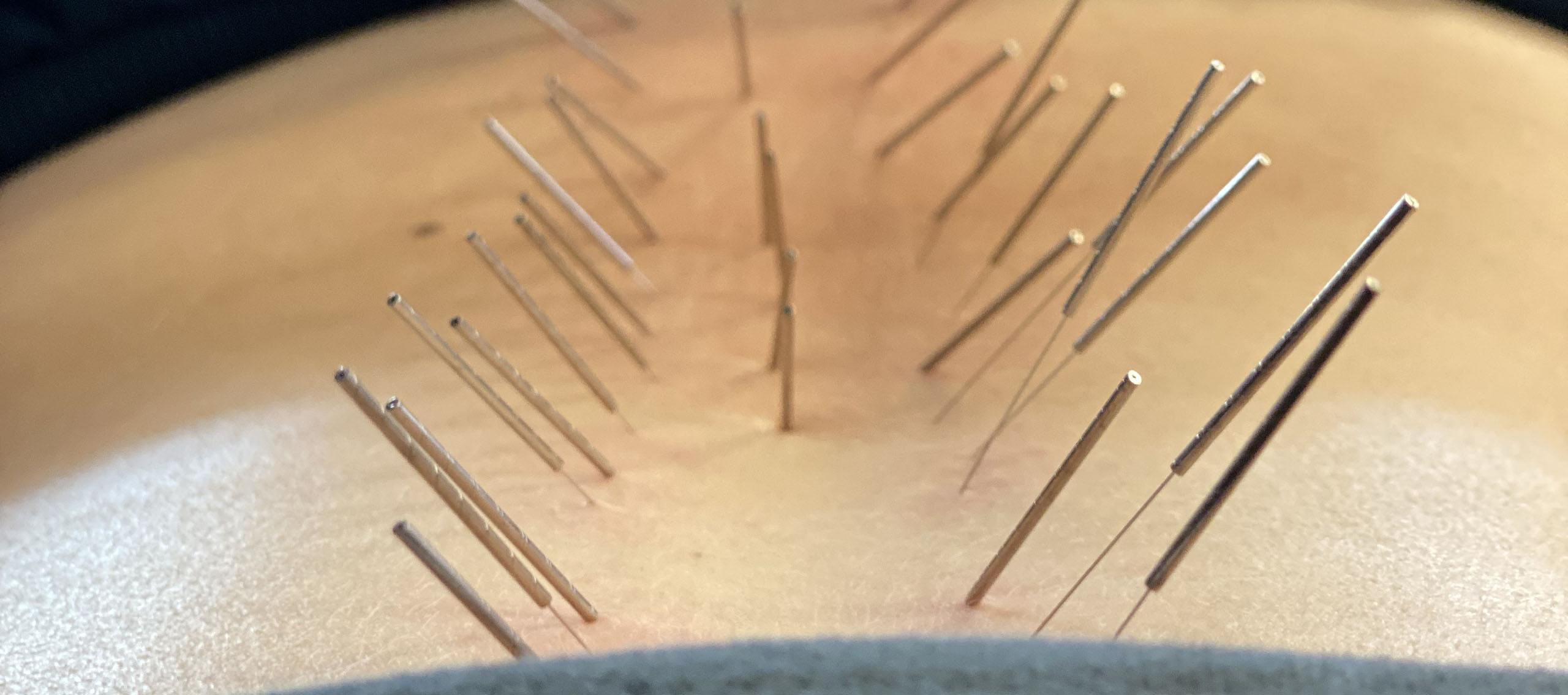
Electrical Auricular Vagus Nerve Dry Needling
The human body and mind respond to physical and emotional stress, trauma, pain, etc., by elevating sympathetics. Elevated sympathetic activity reciprocally inhibits parasympathetic autonomic nervous system activity. Chronic sympathetic autonomic hyperactivity is detrimental to all aspects of physical and mental well-being. It leads to body-wide inflammation, pain, hyperalgesia, tissue rigidity, hypoxia, hormone imbalance, anxiety, depression, intestinal issues, incontinence, and the list goes on.
The auricular branch of the vagus nerve, cranial nerve X, innervates multiple areas of the ear. This is the most direct, powerful, parasympathetic-specific treatment we can perform. The Vagus nerve is sometimes referred to as the “wandering nerve” because of its meandering path leading from the brainstem to the heart, organs, intestines, aorta, and more. It innervates a lot more unique structures than any other nerve I’m aware of. It is the primary nerve of the parasympathetic nervous system and about 80% of its fibers are afferent, indicating its main job is to tell the brain what is going on with our organs and other subconscious bodily functions, so our brain can monitor and regulate what is going on. The vagus nerve gives our brain interoceptive awareness, or awareness of what is going on inside our body.
Sympathetic-dominant autonomic activity, our fight-or-flight response, is only potentially beneficial for human well-being in short durations. A few minutes to a few days, at most. After that, chronic sympathetic autonomic hyperactivity becomes detrimental to all aspects of health. The natural response of the human mind and body to mental or physical stress, strain, pain, or trauma, is to elevate sympathetic activity. Therefore, almost all physical therapy patients present with chronic sympathetic hyperactivity. Almost all psychotherapy patients present this way as well. Most of humanity presents this way, for that matter. This is a massive problem that goes largely unaddressed. To counter this, it is necessary to remove as many sympathetic stressors from the joints and tissues throughout the body as possible (tight, short tissues are pathologic), while concomitantly targeting the parasympathetic autonomic nervous system to further inhibit sympathetic activity and nudge the autonomic nervous system toward homeostasis. A primary factor limiting the amount of treatment an individual can tolerate is how much sympathetic autonomic stimulation you induce while inserting needles.
Each time you get poked with a sharp piece of metal, the sympathetics are stimulated. The more pokes, the more sympathetic stimulation. The more sympathetic stimulation, the more likely you are to push your patient vasovagal. Needle-induced vasovagal response is secondary to sympathetics elevating too much, too fast, for the individual’s threshold, leading to a rapid increase in blood pressure. This stimulates the baroreceptors in the carotids, along with other neurophysiologic processes, and ignites the opposing part of the autonomic nervous system, the parasympathetic autonomic nervous system. The parasympathetics crank up to rapidly depress the sympathetics and protect the mind and body. Targeting the vagus nerve with your first needles helps avoid this as it regulates the autonomic nervous system towards parasympathetic dominance right off the bat.
Remember, the vagus nerve is the primary nerve of the parasympathetic autonomic nervous system. We can most directly stimulate the vagus nerve and parasympathetic activity with electrical auricular vagus nerve stimulation. The homeostatic effect is amplified by connecting the 3 parasympathetic-dominant regions of the body to each other with low-frequency microcurrent, 1-5 Hz (I like 1 Hz because it is closest to a typical heartbeat) – the auricular branch of the vagus nerve in the ear, this is the most direct, the sacral plexus, S2-S4, the suboccipital / C2 spinous process periosteum, the pelvic floor, and the cranial suture lines
Sympathetic-dominant autonomic activity, our fight-or-flight response, is only potentially beneficial for human well-being in short durations. A few minutes to a few days, at most. After that, chronic sympathetic autonomic hyperactivity becomes detrimental to all aspects of health. The natural response of the human mind and body to mental or physical stress, strain, pain, or trauma, is to elevate sympathetic activity. Therefore, almost all physical therapy patients present with chronic sympathetic hyperactivity. Almost all psychotherapy patients present this way as well. Most of humanity presents this way, for that matter. This is a massive problem that goes largely unaddressed. To counter this, it is necessary to remove as many sympathetic stressors from the joints and tissues throughout the body as possible (tight, short tissues are pathologic), while concomitantly targeting the parasympathetic autonomic nervous system to further inhibit sympathetic activity and nudge the autonomic nervous system toward homeostasis. A primary factor limiting the amount of treatment an individual can tolerate is how much sympathetic autonomic stimulation you induce while inserting needles.
Each time you get poked with a sharp piece of metal, the sympathetics are stimulated. The more pokes, the more sympathetic stimulation. The more sympathetic stimulation, the more likely you are to push your patient vasovagal. Needle-induced vasovagal response is secondary to sympathetics elevating too much, too fast, for the individual’s threshold, leading to a rapid increase in blood pressure. This stimulates the baroreceptors in the carotids, along with other neurophysiologic processes, and ignites the opposing part of the autonomic nervous system, the parasympathetic autonomic nervous system. The parasympathetics crank up to rapidly depress the sympathetics and protect the mind and body. Targeting the vagus nerve with your first needles helps avoid this as it regulates the autonomic nervous system towards parasympathetic dominance right off the bat.
Remember, the vagus nerve is the primary nerve of the parasympathetic autonomic nervous system. We can most directly stimulate the vagus nerve and parasympathetic activity with electrical auricular vagus nerve stimulation. The homeostatic effect is amplified by connecting the 3 parasympathetic-dominant regions of the body to each other with low-frequency microcurrent, 1-5 Hz (I like 1 Hz because it is closest to a typical heartbeat) – the auricular branch of the vagus nerve in the ear, this is the most direct, the sacral plexus, S2-S4, the suboccipital / C2 spinous process periosteum, the pelvic floor, and the cranial suture lines
- Concha
- Cymba Concha
- Inner Tragus
Targeting the parasympathetic autonomic nervous system with needles has a homeostatic effect via sympathetic depression and other neurophysiologic mechanisms. We know little about the mechanisms of action that lead to this, but we do know the overall result of vagus nerve and parasympathetic-specific needling. There is good research looking at surgical vagus nerve stimulation with a metal cuff placed around the nerve itself, and good needling research looking at vagus nerve stimulation via its auricular distribution in the ear. A recent meta-analysis found high efficacy in depressing sympathetic hyperactivity and bringing the autonomic nervous system towards homeostasis in both auricular stimulation and surgical implantation. Obviously better to avoid surgery. It is already FDA approved for epilepsy, anxiety and depression. People are looking at its effect on anxiety, chronic pain, autoimmune disease, insulin resistance, heart irregularities, mental health, and much more.
The amount of research on vagus nerve stimulation has significantly increased over the last few years, which is awesome. Hopefully this becomes a widespread treatment for a multitude of mental and physical impairments. It certainly should be. Auricular electrical vagus nerve needling consistently produces incredibly powerful results for my patients and in the literature. An awesome thing about this is it works for a massive range of medical impairments. All conditions do better with a more homeostatic autonomic nervous system, and stimulating the vagus nerve is the most powerful place we can access with needles to directly induce autonomic homeostasis. It helps regulate the primary axes of the autonomic nervous system, including the trigeminocervical complex, which when sympathetically hyperactive causes widespread dysfunction in numerous brain regions, including the locus coeruleus, a main epinephrine producer. Remember, the gut plays a large role in regulating mood, serotonin, dopamine, and immunity, and we are learning more and more about how important it is all the time. Don’t forget, the vagus nerve is the primary nerve connecting the intestines directly to the brain.
To maximize treatment efficacy and homeostasis, it is important to include low-frequency (1-5 Hz) microcurrent. It really helps. Remember, don’t use regular stim with needles, people will not like you, it hurts, too many amps. 1-5 Hz has consistently been shown across electrical research to be the best frequency to stimulate endogenous opioid (beta endorphin) release from the hypothalamic-pituitary-adrenal axis and elsewhere. Up to 4 times the amount of beta endorphin becomes accessible when using low-frequency stim with your needles. Beta endorphin reduces pain, which alone reduces sympathetic activity. It also has other mechanisms of action that we are trying to understand. I like to use 1 Hz microcurrent, closest to normal heartbeat, and connect the parasympathetic dominant areas of the body to each other, in various ways. The other 2 parasympathetic-dominant locations to access with electrical dry needling are the sacral plexus, S2-S4, and the suboccipital periosteum / C2 spinous process, along with the cranial suture lines, which contain active stem cells throughout life (this is a recent discovery). Placing current through periosteal tissue and needling bilaterally are things I do as often as possible. It appears to induce a more powerful homeostatic, healing effect.
I place 3 needles in each ear, or in one ear if that is all I can access, as the first needles of my treatments. I then place needles in the other areas I mentioned above, depending on patient position. Then I treat whatever else I am going to, hook up the microcurrent, and leave the patient for around 60 minutes, if tolerable.
Auricular Vagus Nerve Needling IS NOT Acupuncture
Check out my YouTube Video on this topic:
This irritates me to no end. There is a simple issue that physical therapy boards across the country fail to understand. Acupuncture boards have zero ability to dictate what physical therapists can and cannot do. Unless the PT boards don’t stand up for their practitioners, which is unfortunately common, and is why numerous states told their PT’s they cannot needle. At the moment, only HI, OR, CA, and NY still restrict needling for physical therapists. Numerous states have in their dry needling statute that we cannot use “auricular points.” That DOES NOT mean we cannot needle the ear. I have no idea what auricular points are, what meridians they correspond to, or how to go about determining which points to use. Therefore, it is impossible for me to use “auricular points.” This is like throwing into the physical therapy practice act, oh, and by the way, no breast augmentations or rhinoplasties.
The astounding arrogance of a medical field to attempt to claim sole right to treat a certain area of the body is childish, petty, and pathetic. Don’t get me wrong, I love acupuncture, I think it is awesome. It is also completely different from dry needling. Imagine a physical therapist telling a plastic surgeon they cannot treat the legs because we do that, and physical therapy has certainly been around a lot longer than plastics. Never mind the fact that we perform completely different techniques to help our patients, for different reasons.
In Acupuncture-Land, there are about 360 predetermined points throughout the body. These points correlate with the theory of 12 major meridians, energy lines, 6 Yin & 6 Yang, flowing throughout our body. There is a lot more to it than that, but that’s about what I know. Mind you, a major problem with acupuncture is the entire theory it is based on has never been proven to exist. Now, we have to have energy lines flowing throughout our body. Otherwise, we are dead. They are certainly on to something, which is obviously a reason why acupuncture has persisted through millennia. Acupuncturists somehow determine which meridians are out of balance and they somehow use a combination of points throughout the body to regulate the energy fields. Acupuncture was developed far before we even knew what a cell or a germ was. This is why western medical science is not a part of traditional Chinese medicine. Again, I totally love acupuncture and want to learn more about it.
When acupuncturists needle the ear, they are doing so because there are a lot of acupuncture points in the ear they use to balance various meridians and treat various joints. I do not know the first thing about how they go about doing that. Physical therapists needle the ear because it is innervated by the auricular branch of the vagus nerve, the primary nerve of the parasympathetic autonomic nervous system. An objective fact. Remember, anatomy and physiology are not a part of traditional Chinese medicine and are just touched upon in acupuncture school. Much of their time is spent learning how to think about the meridian system and how to balance them using various Eastern medical techniques that are never discussed in doctoral physical therapy programs.
Anybody can poke someone with a needle, that takes zero skill. I can teach a five-year-old how to poke someone with a needle in 1 minute. The challenge is acquiring the background knowledge necessary to formulate a treatment strategy to be safe and effective. 100% of what PT’s do with needling and other treatment is specifically based on our best objective knowledge of the neuromusculoskeletal systems, which we spend an entire doctorate degree obtaining in the classroom and in the clinic. Zero percent of what acupuncturists do is based on the neuromusculoskeletal systems. Again, when acupuncture was created, nobody knew what a neuromusculoskeletal system was, or if they even existed. They are basing their treatments off of Eastern Medicine, something PT’s get zero exposure to in school.
The whole argument that physical therapists are performing acupuncture simply by having an acupuncture needle in their hand is an incoherent, self-deprecating argument by acupuncturists. If I, having never taken an acupuncture class, am somehow able to perform acupuncture simply by holding a needle in my hand and stabbing someone, every single acupuncture school in the world should be closed, immediately, because you just admitted to me as the basis of your argument that your entire education is useless, by saying someone who has never taken acupuncture courses can perform acupuncture. This is obviously absurd. It is exactly like giving a radiologist a scalpel and telling them they are performing rhinoplasties if they are hacking apart someone’s nose, even though they have zero idea how to perform a rhinoplasty. The scalpel is not special. Anyone can chop up someone’s nose. The knowledge behind how to use it to achieve a certain goal is.
The auricular vagus nerve distribution is the most powerful single location medical practitioners have at their disposal to regulate the autonomic nervous system toward homeostasis, without accessing the nerve itself with a surgical procedure. All medical practitioners should be aware of this and should utilize the ear to help depress sympathetics, elevate parasympathetics, and nudge the mind and body toward homeostasis. This will make every other medical treatment you perform that much more effective. As far as I know, all mental and physical health impairments improve with a more homeostatic autonomic nervous system.

How to Limit Initial Sympathetic Autonomic Stimulation Following Needle Placement to Avoid Vasovagal Response and Patient Discomfort During Dry Needling Treatment
Immediately upon needle insertion, the sympathetic autonomic nervous system elevates above baseline for somewhere around 15 minutes. This has been confirmed in numerous studies using microneurography (gold standard ANS test) and heart rate variability (HRV). HRV is less precise, but cheaper and easier to perform. Lots of watches and even some beds have HRV capability nowadays. Acupuncturists figured out this relationship thousands of years ago by trial and error, and is one of the reasons why acupuncturists typically leave the needles in for somewhere around 30 minutes. Needling works better this way, and the western medical reason behind this is secondary to how our nervous systems respond to needling. Leaving the needles in the body while the nervous systems go from initial sympathetic elevation to parasympathetic dominance significantly improves the overall homeostatic effect of treatment. For many reasons, including the corrupt, pathetic insurance reimbursement, or lack thereof, for treatment, practitioners generally disregard this phenomenon. Big mistake.
If you are working with patients presenting with significant sympathetic hyperactivity (anxiety, fibromyalgia, hyperalgesia, fear of needles, etc.), the initial sympathetic spike from getting poked with a sharp object is often enough to stimulate a vasovagal response. Vasovagal response and syncope are results of sympathetic autonomic stimulation occurring faster than the individual’s system can account for. At a certain sympathetic threshold (everyone is different), the baroreceptors in the carotid and elsewhere become stimulated from a rise in blood pressure. The autonomic nervous system overreacts and turns on the parasympathetic full blast. This reciprocally inhibits sympathetic tone, drops blood pressure, and in extreme cases, can cause fainting. In almost ten years of practice, I have never had a patient pass out on me.
Large nervous system responses are typically more easily elicited in highly athletic and or significantly messed up patients. “Significantly messed up” is a medical term, by the way… Therefore, if you are dealing with a patient presenting with initial, significant sympathetic hyperactivity, their threshold to reach vasovagal response is diminished. In these circumstances, just a few needles can create an uncomfortable situation for the patient. This is why you should always treat people in a position that, if they were to faint, they would not hit their head or something on the way down. This is the main safety issue with vasovagal responses. The response itself is not dangerous, it’s kinda like an autonomic reset, however, it is super uncomfortable and is not something you want to do to your patients.
OK, so, if you are treating someone with hyperactive sympathetics, which covers about everyone we treat, and we know from clinical experience and research that sympathetics spike for about the first 15 minutes following needle insertion before dropping below baseline, it makes sense to target the parasympathetics with the first needles you place. This dramatically limits the amplitude and duration of the sympathetic spike, making treatment more comfortable and effective. I have never read a research article looking specifically at this effect, but I can tell you with absolute certainty that targeting the parasympathetics alone, or in conjunction with and prior to your other needles, limits the sympathetic spike and makes everything better; Pain, speed of outcome, and overall treatment efficacy. This is why you should always try to leave the needles in for about 60 minutes, if possible. Allowing the autonomic transition from sympathetic to parasympathetic dominance while the needles are in the body provides a far more effective, comfortable, and lasting treatment than simply poking someone for a few seconds then removing the needles.
Along with the HPA axis and the trigeminocervical complex, another main portion of the ANS is the gut-brain axis, the brain and the enteric nervous system, which are connected by the vagus nerve. Behind the brain, the enteric nervous system has the largest number of neurons per system in our body. This should indicate its importance and we are learning rapidly the vast potential a homeostatic enteric nervous system has on our general well-being. The intestines and their microbiota play an important role in many bodily functions, including our immune system, hormones, neuromodulators, mental state, and more. Sympathetic hyperactivity negatively affects the gut-brain axis, limits gastric motility, disrupts serotonergic and dopaminergic neuronal activity, sense of wellbeing, and a lot more bad stuff. Remember, we have more microbiome bacteria than we do cells in our body. Yes, this means we have more aliens in our body than we do our own cells. Whose really running the show here? Seriously.
By specifically targeting the parasympathetics first, then proceeding with your other needling treatment, you will limit the initial sympathetic spike from being poked by needles by initially stimulating the opposing portion of the autonomic nervous system. Limiting the duration and amplitude of the sympathetic spike reduces the likelihood of vasovagal response, improves patient comfort, and gives the patient more time with the needles in the body while the parasympathetics are dominant. This is key. It maximizes homeostasis, treatment efficacy, and patient outcome.
Should I Utilize the “Pistoning” Technique While Performing Dry Needling?
Sympathetic-dominant autonomic activity, our fight-or-flight response, is only potentially beneficial for human well-being in short durations. A few minutes to a few days, at most. After that, chronic sympathetic autonomic hyperactivity becomes detrimental to all aspects of health. The natural response of the human mind and body to mental or physical stress, strain, pain, or trauma, is to elevate sympathetic activity. Therefore, almost all physical therapy patients present with chronic sympathetic hyperactivity. Almost all psychotherapy patients present this way as well. Most of humanity presents this way, for that matter. This is a massive problem that goes largely unaddressed. To counter this, it is necessary to remove as many sympathetic stressors from the joints and tissues throughout the body as possible (tight, short tissues are pathologic), while concomitantly targeting the parasympathetic autonomic nervous system to further inhibit sympathetic activity and nudge the autonomic nervous system toward homeostasis. A primary factor limiting the amount of treatment an individual can tolerate is how much sympathetic autonomic stimulation you induce while inserting needles.
Each time you get poked with a sharp piece of metal, the sympathetics are stimulated. The more pokes, the more sympathetic stimulation. The more sympathetic stimulation, the more likely you are to push your patient vasovagal. Needle-induced vasovagal response is secondary to sympathetics elevating too much, too fast, for the individual’s threshold, leading to a rapid increase in blood pressure. This stimulates the baroreceptors in the carotids, along with other neurophysiologic processes, and ignites the opposing part of the autonomic nervous system, the parasympathetic autonomic nervous system. The parasympathetics crank up to rapidly depress the sympathetics and protect the mind and body. Targeting the vagus nerve with your first needles helps avoid this as it regulates the autonomic nervous system towards parasympathetic dominance right off the bat.
Remember, the vagus nerve is the primary nerve of the parasympathetic autonomic nervous system. We can most directly stimulate the vagus nerve and parasympathetic activity with electrical auricular vagus nerve stimulation. The homeostatic effect is amplified by connecting the 3 parasympathetic-dominant regions of the body to each other with low-frequency microcurrent, 1-5 Hz (I like 1 Hz because it is closest to a typical heartbeat) – the auricular branch of the vagus nerve in the ear, this is the most direct, the sacral plexus, S2-S4, the suboccipital / C2 spinous process periosteum, the pelvic floor, and the cranial suture lines.
What is Pistoning?
The pistoning technique involves a sewing-machine, or jack-hammer-like motion of the needle going in and out of tissue. The needle is not fully removed from tissue following initial insertion, but is poked in and out of the tissue at varying angles, with the goal of puncturing numerous locations of trigger points (hypercontracted groups of sarcomeres), or other pathologic tissue. Once the poking is done, the needle is removed. Get your mind out of the gutter people! There is also no twisting of the needle and there is no electrical stimulation applied. This is how the majority of practitioners dry needle in the United States. This approach to needling completely disregards the neurophysiologic state of our patient’s mind and body. Sympathetic autonomic nervous system hyperactivity.
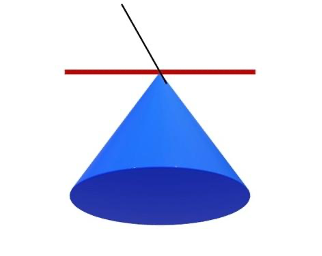
For this discussion, let’s define pistoning as 10 advances of a 0.2 mm diameter needle into an area of tissue in a cone shape. Following the 10 advances, the needle is removed. This is common practice in Pistoning-Land. In the image below, think of the red line as the skin, the black line is the needle, and the blue cone is the tissue the needle is inserted into. Advancing a 0.2 mm diameter needle 10 times allows you to treat a cone of tissue with a circular base of tissue with a 2 mm diameter (0.2 mm x 10 = 2 mm). A circle with a 2 mm diameter has an area of 3.14 mm2. So, the bottom of the cone is about 3.14 mm2 with a diameter of 2 mm.
I am just going to refer to the area of the base of the cone for comparison, but keep in mind the area is much greater, as it included the entire cone, which depends on the length of the needle and the depth you insert the needle.
Note: The math in this article is as accurate as I could get without more advanced equipment. I am sure this is off a bit, but I think it conveys the general state of matters. I would love any thoughts on this from fellow needlers or if anyone has more data on this.
Should I Piston?
The short answer? No, you should not, in general. Now, there are instances when pistoning is useful. However, it should only be utilized in specific circumstances, with specific patients. There are numerous reasons for this. You should never piston over the lung field, bladder, or anything else you do not want to hit. In reality, you can likely hit just about anything in the body 1 time with a solid, 0.2 mm thick piece of surgical steel and never cause any noticeable damage. You would probably never know anything happened. Remember, normal, hollow needles are typically somewhere between 1-2 mm, and those are stuck into vessels, organs, etc., all the time, on purpose, without a problem. Hitting something more than once in a small area is when you run an increased risk of significant damage. Not pistoning decreases risk, as far as vasovagal response and tissue damage goes, and improves treatment efficacy via improved autonomic nervous system homeostasis. Remember, MD’s take chunks out of organs to biopsy them in the lab. The intestines and heart can be sutured, the lungs and bladder can be drained. All this is done with needles or cutting instruments exponentially larger than the tiny, solid, 0.2 mm, or so, thick needles we utilize. Aside from increasing safety and efficacy, not pistoning is exponentially more comfortable for the patient, part of the reason it increases efficacy. This is important.
Pistoning, in general, makes negative neurophysiologic sense. One goal of dry needling should be removing as much pathologic tissue throughout the body as possible, with the express intent of depressing sympathetics, elevating parasympathetics, and guiding the autonomic nervous system toward homeostasis, allowing the body to heal the mind and the mind to heal the body. This involves treating the body as an intricately interconnected complex system.
If a pistoner inserts a single needle 10 times into the same small area of tissue, you are stimulating the sympathetics 10 times. If you use 2 needles, that is 20 times the sympathetics are stimulated in the process of treating an overall area of (3.14 mm2 x 2) 6.28 mm2 of tissue.
Now, take a peek at the ultrasound image below from Helen Langevin, MD, a world-renowned researcher on the effects of acupuncture on soft tissue. The black dot in the left picture is a needle placed in tissue without rotating it. I look at this as the needle treating only the tiny bit of tissue it is through, the black dot. The picture on the right is after the needle has been rotated until it won’t turn anymore. In this picture, the entire square, and more, is being treated. I copied the scale bar and imposed it over the right picture in the blue lines. Each of the blue lines is 1 mm. There are 7 lines there. So, the area of each square image is about 7 mm x 7 mm = 49 mm2.
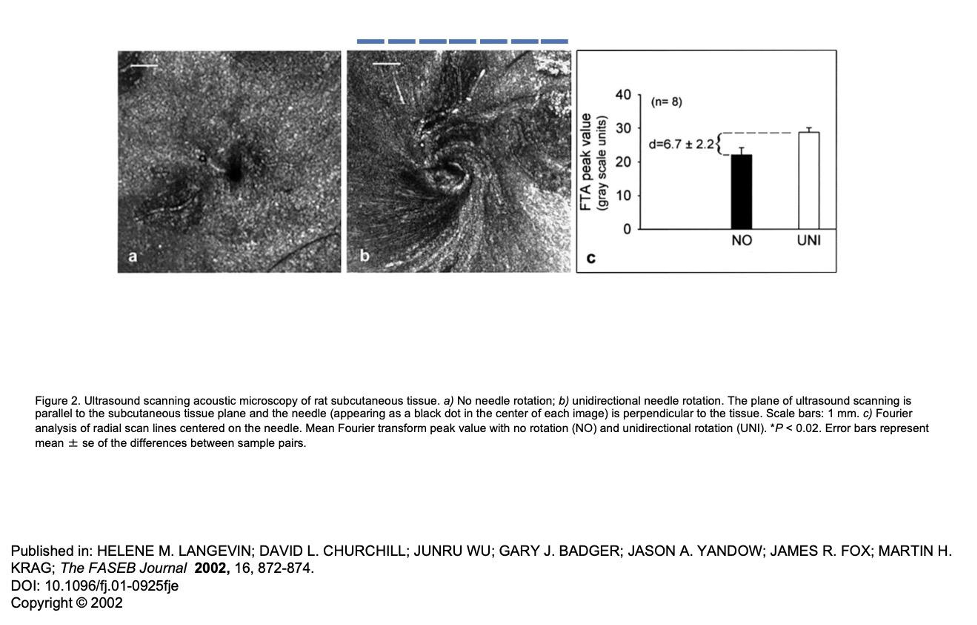
By inserting 2 needles, one time each, and rotating them until tight (49 mm x 2), you treat about 98 mm2 of tissue with 2 needle advances, or two stimuli to the sympathetics. Compare this to pistoning, where with 2 needles, advancing each 10 times into tissue, without needle rotation, you elicit 20 stimuli to the sympathetics, while only treating 6.28 mm2 of tissue. So, with pistoning, you are eliciting 10 times the sympathetic autonomic stimulation and treating about 15 times less tissue area (98 mm2 / 6.28 mm2 = 15). Which one seems better to you?
Furthermore, if you advance each needle once and twist it, rather than 10 times each without twisting, you can use 20 needles to treat 980 mm2 of tissue (156 times more tissue) and stimulate the sympathetics the same amount as if you pistoned with only 2 needles, covering only 6.28 mm2 of tissue (980 mm2 / 6.28 mm2 = 156).
Pistoning Technique
2 needles x 10 needle advances per needle = 20 sympathetic stimuli covering 3.14 x 2 mm2) = 6.28 mm2 of tissue.
Intricate Art single Advancement Technique
20 needles x 1 needle advance per needle = 20 sympathetic stimuli covering 20 x 49 mm2 = 980 mm2 of tissue.
980 mm2 divided by 6.28 mm2 = 156. That means, with 1 advancement into tissue per needle, you can treat 156 times the area of tissue with the same amount of sympathetic stimulation as compared to pistoning. Or, you can treat the same amount of tissue area with our technique as with pistoning, while stimulating the sympathetics 16 times less (49 mm2 / 3.14 mm2 = 15.6). Again, the goal is to treat as much pathologic tissue with as little sympathetic stimulation as possible. The end-goal is sympathetic depression, parasympathetic elevation, and autonomic homeostasis, so which technique is more logical? The last thing physical therapy patients need is more sympathetic autonomic hyperactivity. They are already suffering, at least in part, specifically because they are living with chronic sympathetic autonomic nervous system hyperactivity.
The only time I will utilize pistoning is if I get a twitch out of the muscle and I want to see if I can elicit more twitches. If not close to anything I do not want to poke. When you hit a knot with a needle, a lot of the time you elicit a twitch, or muscle contraction. This is secondary to a bunch of sarcomeres unlocking from each other. This is the fastest way to dissolve trigger points. It can really hurt, which is not a negative thing, however, it will scare a lot of people away from future needling treatment, depending on their personality. I almost never piston, and I definitely do not piston the first few treatments when introducing a patient to needling, and you should never do pistoning if the patient is hypersensitive or prone to vasovagal response, as this significantly amplifies pain and sympathetic stimulation. Again, our patients live with chronic sympathetic hyperactivity. Everything in our power should be done to depress sympathetics and elevate parasympathetics, leading the autonomic nervous system toward homeostasis. The key to health.
Thanks for reading. Let me know if anyone has any questions about anything. Talk to you soon.
Jason
DISCLAIMER: The content on the blog for Intricate Art Spine & Body Solutions, LLC is for educational and informational purposes only, and is not intended as medical advice. The information contained in this blog should not be used to diagnose, treat or prevent any disease or health illness. Any reliance you place on such information is therefore strictly at your own risk. Please consult with your physician or other qualified healthcare professional before acting on any information presented here.
References
Butts, R., Dunning, J. and Serafino, C., 2021. Dry needling strategies for musculoskeletal conditions: Do the number of needles and needle retention time matter? A narrative literature review. Journal of Bodywork and Movement Therapies, 26, pp.353-363.
Kearns, G.A., Brismée, J.M., Riley, S.P., Wang-Price, S., Denninger, T. and Vugrin, M., 2022. Lack of standardization in dry needling dosage and adverse event documentation limits outcome and safety reports: a scoping review of randomized clinical trials. Journal of Manual & Manipulative Therapy, pp.1-12.
Padanilam, S.J., Dayton, S.R., Jarema, R., Boctor, M.J. and Tjong, V.K., 2021. Dry Needling for the Treatment of Musculoskeletal Ailments With Trigger Points. Video Journal of Sports Medicine, 1(5), p.26350254211023776.
Butt, M.F., Albusoda, A., Farmer, A.D. and Aziz, Q., 2020. The anatomical basis for transcutaneous auricular vagus nerve stimulation. Journal of anatomy, 236(4), pp.588-611.
Garner, B.K., Hopkinson, S.G., Ketz, A.K., Landis, C.A. and Trego, L.L., 2018. Auricular acupuncture for chronic pain and insomnia: a randomized clinical trial. Medical acupuncture, 30(5), pp.262-272.
Jaić, K.K., Turković, T.M., Pešić, M., Djaković, I., Košec, V. and Košec, A., 2019. Auricular acupuncture as effective pain relief after episiotomy: a randomized controlled pilot study. Archives of gynecology and obstetrics, 300(5), pp.1295-1301
Johnson, R.L. and Wilson, C.G., 2018. A review of vagus nerve stimulation as a therapeutic intervention. Journal of inflammation research, 11, p.203.
Kang, H.R., Lee, Y.S., Kim, H.R., Kim, E.J., Kim, K.H., Kim, K.S., Jung, C.Y. and Lee, J.K., 2017. A clinical study of electroacupuncture and auricular acupuncture for abdominal pain relief in patients with pancreatitis: A pilot study. Korean Journal of Acupuncture, 34(1), pp.47-55.
Moura, C.D.C., Chaves, E.D.C.L., Cardoso, A.C.L.R., Nogueira, D.A., Azevedo, C. and Chianca, T.C.M., 2019. Auricular acupuncture for chronic back pain in adults: a systematic review and metanalysis. Revista da Escola de Enfermagem da USP, 53.